The influence of gut bacteria on obesity
- Amanda Cleghorn
- May 17, 2016
- 5 min read
Updated: Jan 11
Gut bacteria are currently the subject of a huge amount of exciting and ground breaking research. Scientists are now able to analyse hundreds of what are possibly a thousand or more species which live inside your body, making up your personal microbiome. They are beginning to piece together the evidence which links certain gut bacteria profiles to various disease states, or potential risk of disease, such as cancer of the colon, autoimmune diseases, or autism.
Obesity is now classed as a disease and research indicates that gut bacteria may be heavily implicated in its development, through various mechanisms associated with extraction of energy from food; inflammation; insulin sensitivity; and communication with the brain.
Energy harvesting
One of the many functions that beneficial bacteria carry out in the digestive tract is fermentation of indigestible dietary fibre, which produces short chain fatty acids (SCFAs), such as butyrate. Butyrate is a vital source of energy for the cells which line the colon. In other words, the combination of beneficial gut bacteria and a high fibre diet are of great importance for digestive health, as the fatty acids which are produced nourish the colon cells. It is therefore not surprising that low concentrations of SCFAs have been found in patients with inflammatory bowel diseases such as ulcerative colitis, along with very different bacterial profiles to healthy subjects. Studies on both mice and humans have shown that obese individuals have a different microbiome (mixture of bacterial species) to lean individuals and it seems that the gut bacteria in obese subjects are more efficient at extracting, or ‘harvesting’ energy from the diet.
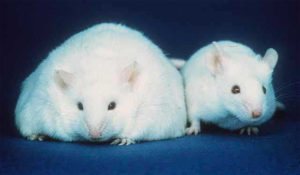
Many studies use germ-free mice, animals which are born by caesarean section with a sterile gut and kept in a sterile environment. It has been found that germ-free mice remain leaner than conventionally raised mice, even when eating a higher intake of food. If bacteria from normal mice are introduced into germ-free mice, their body fat has been found to increase by 60% in just 10-14 days, even when food consumption is reduced. Germ-free mice which were colonised with bacteria from obese mice saw significant increases in body fat, which supports the idea that certain types of bacteria are able to release more energy from food - energy which is then stored as fat.
Inflammation and insulin sensitivity
Butyrate, the SCFA produced by bacterial fermentation of fibre, is important for helping to keep the lining of the digestive tract intact. A healthy mucosal lining ensures that partially digested food and bacteria does not pass through from the inside of the gut, into the bloodstream. Any disruption to the balance of bacteria in the gut can cause inflammation, which damages the delicate lining and enables unwanted substances to find their way into the blood, where they trigger an immune response. Further inflammation ensues.
Obesity is understood to be driven by inflammation, partly because the production of inflammatory chemicals by the immune system causes cells to become less sensitive to insulin. If we consume food which is converted to glucose and cells do not respond to insulin, (i.e. they are resistant to insulin, which attempts to direct glucose into cells to be used as energy), then rather than allow blood levels of glucose to become dangerously high, the body converts glucose to fat and stores it, leading to weight gain.

Faecal transplants are becoming more commonly used in medical procedures on humans, as well as mice, although trials are typically small in size (possibly due to lack of volunteers?!) One small study analysed men who were insulin resistant (a condition which typically precedes the development of type II diabetes) and who received a faecal transplant from lean donors. Those who received the transplant were observed to have a significantly improved sensitivity to insulin, as well as a much more diverse range of bacteria in their gut, particularly the species which produce the beneficial butyrate SCFA.
Communication with the brain
Gut bacteria have been shown to influence the neurotransmitter (brain chemical) serotonin , of which 90% is actually produced in the gut. Serotonin plays a major role in regulating our mood, as well as our appetite and feeling of satiety, or feeling full after eating. It helps to regulate insulin production by the pancreas and is therefore implicated, in partnership with gut bacteria, in the progression of insulin resistance towards diabetes and obesity.
Dietary and lifestyle measures to address the obesity crisis
Obesity is a complex disease, but what is becoming clearer, is that inflammation is at the heart of the issue and that the microbiome has an influential role. It is therefore crucial to minimise dietary and lifestyle factors which contribute to inflammation, such as sugar, wheat, processed vegetable oils and artificial additives, as well as stress, poor sleep, antibiotics (which disrupt the beneficial gut bacteria), or environmental toxins such as fertilisers and pollution.


Probiotic supplements can be an effective way to colonise the gut with beneficial bacteria and natural ways to boost your levels of bacteria include eating prebiotic fibre such as onions, garlic, leek, chicory, asparagus and banana, as well as fermented food and drinks such as kefir, kombucha, sauerkraut and tempeh.



Zhang Y-J, Li S, Gan R-Y, Zhou T, Xu D-P, Li H-B (2015) Impacts of gut bacteria on human health and diseases. International Journal of Molecular Science, 16 (4): 7493-7519. PubMed Central (http://www.ncbi.nlm.nih.gov/pmc).
Sansonetti P J, Medzhitov R (2009) Learning tolerance while fighting ignorance. Cell, 138 (3): 416-420. Cell (www.cell.com).
Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková H, Rossmann P, Bártová J, Sokol D, Funda DP, Borovská D, Reháková Z, Sinkora J, Hofman J, Drastich P, Kokesová A (2004) Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology Letters, 93 (2-3): 97-108. PubMed (http://www.ncbi.nlm.nih.gov/pubmed).
Sekirov I, Russell S L, Antunes C M, Finlay B (2010) Gut microbiota in health and disease. Physiological Reviews, 90 (3): 859-904. Physiological Reviews (http://physrev.physiology.org).
Sharma V, Garg S, Aggarwal S (2013) Probiotics and liver disease. The Permanente Journal, 17 (4): 62-67. PubMed Central (http://www.ncbi.nlm.nih.gov/pmc).
Hartstra A V, Bouter K E C, Backhed F, Nieuwdorp M (2015) Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care, 38 (1): 159-165. Diabetes Care (http://care.diabetesjournals.org).
Tsai F, Coyle W J (2009) The Microbiome and Obesity: Is Obesity Linked to Our Gut Flora? Current Gastroenterology Reports, Sep: 307-313. ResearchGate (https://www.researchgate.net).
Hotamisligil G S, Shargill N S, Spiegelman B M (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science, 259 (5091): 87-91. PubMed (http://www.ncbi.nlm.nih.gov).
Vrieze A, Van Nood E, Holleman F, et al. (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology, 143: 913–916. CaelusHealth (http://caelushealth.com).
Oh C-M, Park S, Kim H (2016) Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes Metabolism Journal, 40 (2): 89-98. PubMed Central (http://www.ncbi.nlm.nih.gov/pmc).





Kommentare